Frank Soldano's life has been a rollercoaster of medical challenges, resilience, and hope. For over two decades, he has battled the debilitating effects of inflammatory bowel disease (IBD), a condition that has taken him through hundreds of treatments, emotional struggle, and a relentless search for a better quality of life. At 46, Frank finds himself once again in a pivotal moment — this time as a participant in the groundbreaking BOOM-IBD clinical trial.
The Early Years: A Life Turned Upside Down
Frank was first diagnosed with ulcerative colitis in the late 1990s, a period when understanding and treatments for IBD were far from where they are today. Fresh out of college, Frank was just beginning to navigate adulthood when his life took a drastic turn. "I use the restroom," he recalls, "I'm like, what's going on? I can't stop going, dozens of times a day...I'm thinking I’m Italian, maybe it's just undigested tomatoes."
But it was more than just tomatoes. A trip to the hospital and his first colonoscopy confirmed his worst fears. At just 21, Frank was thrust into the maze of medical care, endless testing, and a grim list of dietary restrictions. "Your whole life gets turned upside down," he says. "No more drinking alcohol, no more spicy foods, no more dairy, no more this, no more that, no more normal."
It's no surprise that Frank's journey through the healthcare system was fraught with frustration. Emergency room visits became a regular part of his life, yet even the doctors seemed uncertain about how to treat him. "No emergency room doctor knew really what to do with the symptoms that I would show up with," he says. "They still don't know what really causes it."


Decades of Struggle and Determination
Over the years, Frank tried every medication available, from steroids to biologics like adalimumab [sold with the brand name Humira] and tofacitinib [sold as Xeljanz], each with its own set of side effects and complications. "I've had skin cancer three times...in my groin area that's never been exposed to sunlight. I have incredible joint pain. People really don’t know the side effects we have to live with," he shares. "I've done Humira, Simponi, Stelara, Xeljanz...and I've been on steroids for years."
It wasn't just the physical symptoms that Frank had to contend with. The emotional and mental toll was just as heavy. The societal lack of support for those with chronic illness is grinding, and endless advocating took over. "I'm tired of fighting for everything I have to do," he says. "My ideal life is to go to work, come home, go to the gym three days a week, have a little bit of time for myself, my wife...I would be happy with that.”
Breakthrough: BOOM-IBD Trial

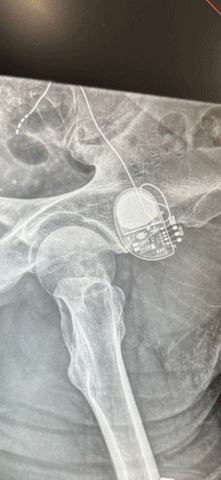
In 2023, after being out of work for several months due to a severe flare-up, late-night scrolling through the internet led Frank to discover the BOOM-IBD clinical trial. This trial, led locally by the IBD Center at Columbia, is exploring a potential novel use of sacral nerve stimulation (SNS) under an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration for treating Crohn’s Disease and ulcerative colitis.
The SNS device is implanted under the skin at the top of the buttocks, where it delivers electrical pulses to stimulate the sacral nerve. Marco B. Zoccali, MD, colorectal surgeon and principal investigator, explains, "By stimulating a sacral nerve root, we can actually trigger the parasympathetic nerves to release impulses that restore balance in the inflammatory versus anti-inflammatory signals in the intestine."
For Frank, this new approach offered a glimmer of hope. "I started bawling crying," he recalls. "I just kept praying that hope I can qualify, and I was doubtful." But qualify he did. Just weeks after receiving the implant, Frank began noticing changes. "Two weeks," he says, "My stool was formed. I was going from four times a day to two times a day."
A New Lease on Life
The Boomerang Medical BOOM-IBD trial has given Frank a chance to reclaim the parts of himself that had been overshadowed by years of illness. "The device is definitely doing what it needs to do," he affirms. "I'm one pill away from being medication-free."
While the journey is far from over, and challenges still lie ahead, Frank remains optimistic about his future and the potential of this device to transform lives. "I’m so passionate about it. I just want to be a part of it, spreading the word," he says. "I want to help the whole country of people that are now getting newly diagnosed."
The Boomerang Medical BOOM-IBD trial represents more than just a new treatment for Frank; it symbolizes hope, resilience, and the power of innovation to really change lives. And for Frank, that possibility is more than enough reason to keep fighting.
More About the BOOM-IBD Trial
The BOOM-IBD trial is a prospective, single-arm, multicenter feasibility trial to evaluate the safety and performance of SNS in patients with Crohn’s disease or ulcerative colitis. The trial is currently closed to enrollment. The SNS device used in this trial is limited by Federal (or United States) law to investigational use. Click here for more information about the BOOM-IBD Trial.
Study Contact:
Call (212) 342-4102
Claudia Musat, MD: cm2065@cumc.columbia.edu

